Yes vinegar is good conductor. Is sugar water a good conductor of electricity.

Does Sugar Solution Conduct Electricity Techiescientist
Hence sugar solution is bad conductor of electricity.
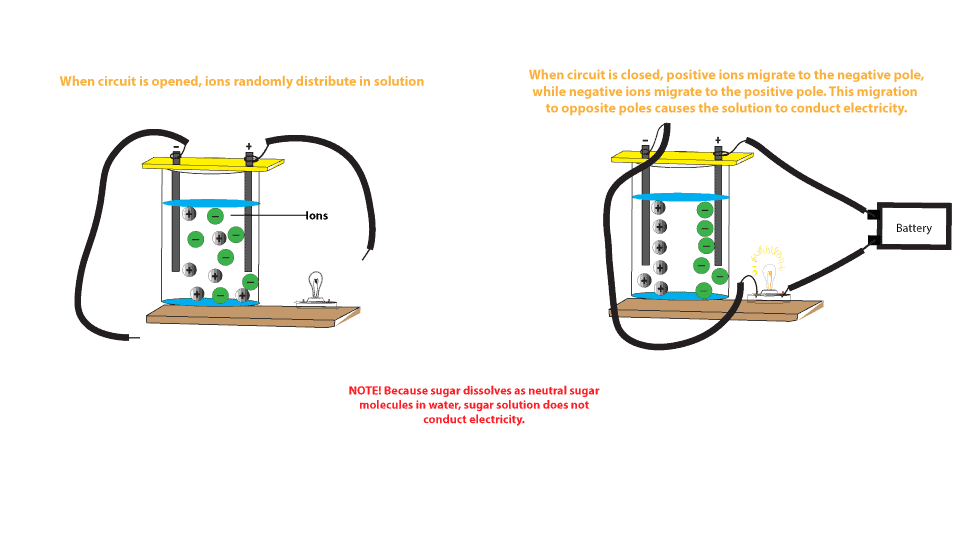
. Yes but no more than distilled water. Distilled water contains only 10-7 molL of H and 10-7 molL of OH-. These sugar molecules are usually neutral not charged and so are unable to move.
A sugar solution is not a good conductor of electricity. A sugar solution is a nonconductor because no ions are present c. Sugar equipment does no contain totally free ions the are compelled to conduct electricity.
Hence solution of sodium chloride is good conductor of electricity. A sugar solution is formed when sugar is dissolved in water. There are no free-to-move electrons in a solution e.
Sugar is a nonconductor. Its those dissolved ions that can conduct well - and when water contains many dissolved ions those ions make a good conductor. Therefore the glucose solution is a non-electrolyte and is a poor conductor of electricity.
But common salt consists of sodium and chloride ions and is formed by the transfer of one electron of sodium to a chlorine atom. Thus a sugar solution contains only neutral molecules of sugar and water. The neutral molecules do not have any charge and thus do not conduct electricity.
Water is a terrible conductor of electricity. On the other hand sugar solution does not conduct an electric current because sugar C 12 H 22 O 11 dissolves in water to produce sugar molecules. Thus pure metals such gold silver and.
It may contain several salts dissolved in it. The water that we get from sources such as taps hand pumps wells and ponds is not pure. Small amounts of mineral salts are naturally present in it.
A solution of cane sugar does not conduct electricity because cane sugar is a covalent compound which is bonded by the sharing of electrons. No sugar solution does not conduct electricity. Small amounts of mineral salts are naturally present in it.
Is sugar solution conduct electricity. This February I will lay out sweet things and renouncing sugar for a whole month to raise funds for research cancer in the. Good insulators are often made of glass plastic rubber ceramic or cloth.
Thank you for visiting my fundraising page. Liquid in which ionic compounds can dissolve easily. Sugar as a non-electrolyte material does not create ions when dissolved in water making it a poor conductor.
In the solution it does not have ions to conduct electrical charges across the solution. Thus sugar solution is a bad conductor of electricity. Furthermore is Tin an insulator or conductor.
If a small amount of sugar is added to distilled water the resulting solution will be a good conductor or poor conductor of electricity. Which of the following is an. A sugar solution comprises sucrose molecules but no ions.
Cations in a solution move toward the positively charged electrode 17. When it dissolves into water it dissolves as a covalent molecule. This water is thus a good conductor of electricity.
When sugar is dissolved in water sugar does not dissociate into ions. That being said water undergoes a process called self-ionization in which. Pure water is actually a good insulator.
This water is thus a good conductor of electricity. It is a non electrolyte. Is glucose a good conductor of electricity When glucose is added to water it readily dissolves but it does not dissociate into ions.
Electricity flows through objects that are conductors and doesnt flow through objects that are insulators. In the water acetic acid dissociates and releases H and CH 3 COO-ions which results in the conductivity due to the migration of ions. Sugar does not ionize when dissolved in water so it does not produce ions that are necessary to conduct electricity.
The sodium chloride solution mainly consists of free sodium and chloride ions which could migrate to positively charged electrodes. Which of the following is not an example of a colloid a. Nonpolar substance d solute.
A salt water solution. A sugar water solution c. A substance whose water solution is a good conductor of electricity is a n a.
QuestionsToggle search form Which substance conductor electricity Posted January 19 2022 Blog Admin Metals can conduct the maximum amount electricity when there resistance. If by sugar you are referring to table sugar sucrose it does not conduct electricity to any appreciable extent when dissolved in water. Good conductors are generally made of metal such as copper aluminum silver gold brass tin and lead.
Street molecules are hosted by covalent bonds as a result they do not dissociate cost-free ions in water. YES it is good conductor if electricity as acid are good conductor of electricity. Is sugar solution a good conductor of electricity Is sugar a good conductor of electricity.
An electric current is a movement of electric charge d. The water that we get from sources such as taps hand pumps wells and ponds is not pure. However water is a great ionic solvent.
Is sugar solution a conductor of electricity. It may contain several salts dissolved in it. Vinegar is aqueous solution of acetic acid.
Unequal electrolytic solution sugar equipment does not dissociate free ions making that an insulator. As a covalent molecule it does not conduct electricity in the way that ionic compounds like salt would.
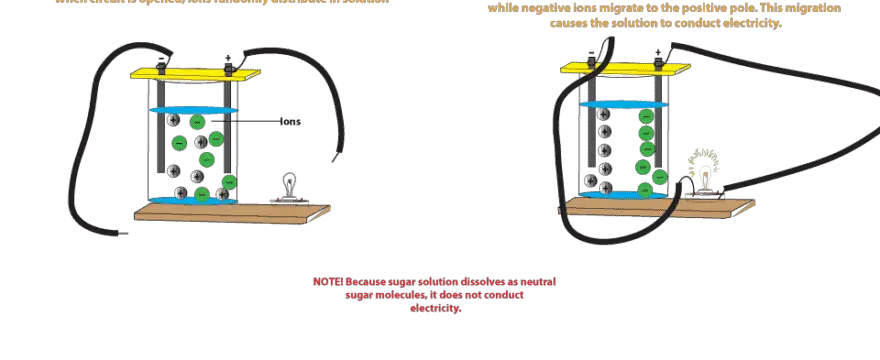
Why Does Salt Solution Conduct Electricity While Sugar Solution Doesn T

Why Does Salt Solution Conduct Electricity While Sugar Solution Doesn T

0 Comments